Although neither atoms nor ions have sharp boundaries they are sometimes treated as if they were hard spheres with radii such that the sum of ionic radii of the cation and anion gives the distance between the ions in a crystal lattice ionic radii are typically given in units of either picometers pm or.
Ceramic ion radius.
But you may remember that i said that ionic radius changes with co ordination.
Ionic crystals cation radius nm anion radius nm 0 100 0 133 0 072 0 14 0 102 0 182 0 053 0 140 0 040 0 140 note.
Radius ratio rule is the ratio of the ionic radius of the cation to the ionic radius of the anion in a cation anion compound.
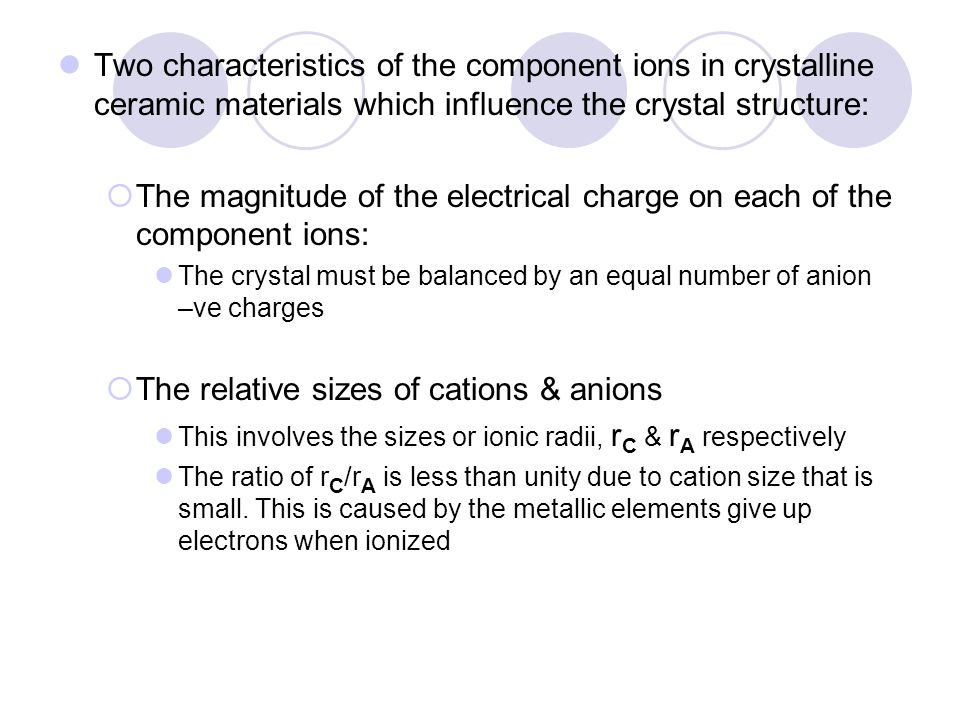
Ionic radius r ion is the radius of an ion regardless of whether it is an anion or a cation.
The ratio of radius of cation to anion is called radius ratio.
3 chapter 11 simple ceramic crystal structures ionic abo 3 perovskite ab 2 o 4 spinel al 2 o 3 corrundum caf 2 fluorite zns zincblende nacl cscl anion coordination.
Larger anion radius most ionic crystals can be considered as close packed structure of anions with cations in the interstitial sites.
The smallest group of particles in the material that constitutes this.
This is simply given by.
Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of three dimensional space in matter.
Calculate the critical minimum radius ratio r r for the triangular coordination cn 3 of three anions of radii r surrounding a central cation of cadius r in an ionic solid.
4 co ordinated nitride ions have a radius of 0 146 nm.
Although neither atoms nor ions have sharp boundaries it is useful to treat them as if they are hard spheres with radii.
In condensed matter physics and inorganic chemistry the cation anion radius ratio also.
In crystallography crystal structure is a description of the ordered arrangement of atoms ions or molecules in a crystalline material.
Ionic radius r ion is the radius of a monatomic ion in an ionic crystal structure.
Ceramic structures two or more different elements more complex than metal structures ionic and or covalent bonds a mix of ionic and covalent bonds.
These ionic radius values are for 6 co ordinated ions with a slight question mark over the nitride and phosphide ion figures.
Values range from 30 pm to over 200 pm.
In this way the sum of ionic radii of a cation and an anion can give us the distance between the ions in a crystal lattice.
Metallic ions positively charged anions.
The ionic radius is half the distance between two gas atoms that are just touching each other.
The present work introduces the concept of shape strain to illustrate its beneficial effect to control avoid any undesired phase transition via cons.
Nitrogen is a particularly good example of this.